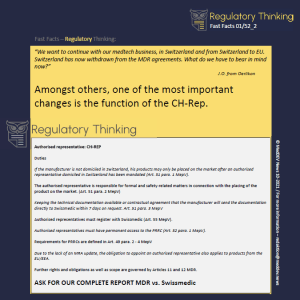
How to start with Regulatory Thinking® ?
#FastFactFriday
To think regulatory, one first needs to know the literature as the master-toolbox. Understanding the Medical Devices Regulation (EU) 2017/745 (MDR) in its smallest fragments is key to using it as an most important strategic tool to enhance one’s business model.
Each Friday we release one fact with the motto:
Guiding you through the MDR, one article at a time.
Follow us on LinkedIn to not miss any #FastFactFriday.

Opinion. New manufacturing methods that promise unimagined freedom in design and development, but also in production and distribution, software to be used as medical application or artificial intelligence as part of IVDs or medical devices often inspire bold ideas: Provision of implants on demand, production of patient-specific implants directly in the operation room, combination of artificial and biological materials, tissue printing up to the printing of whole organs, virtual reality in rehabilitation, software as decission maker or IVDs in a home-care setting.
„It is important to have these visions in mind: This is the only way to free medical device manufacturing from traditional process constraints.“
Dietmar Schaffarczyk, RegulatoryExperts @ ETHzürich
Often, production, working-principles and applications of implants and other medical devices still reminds us of the previous century. There are industries that are already using the Industry 4.0 concept and the use of artificial intelligence far more creatively than medical technology. Medical technology has long been considered a rather cumbersome industry, and interdisciplinary thinking is often still in its infancy. However, medical technology often seems cumbersome for one reason in particular, and that is due to the fact that medical devices have to undergo established and fixed procedures for verification, validation and approval combined with legal restrictions and insurance issues.
„Regulations and legal texts such as standards and norms and/or the Medical Device Regulation (Regulation EU 2017/745) are not written to anticipate safety and performance requirements for future technologies but attempt to describe what is already established in a set of regulations. Innovative technologies are therefore not found in these texts and fall outside the scope.“
To ensure that innovative technologies can still be used in the treatment of patients, it is particularly most important to know exactly the applicable standards and laws. Only in this way it can be ensured that the „spirit“ of the underlying regulation is adhered to, even in the case of new, innovative and promising technologies, and that the requirements of the regulations and laws are also adapted and applied in their “original sense” to new technologies and methods.
The lowest common, but most important denominator must be found here and can be described as follows: to ensure the safety and performance of medical technology at all times by means of a transparent and risk-based approach.
This should be the common thread to follow. Often, this approach slows things down considerably and clips the wings of some overly inspired ideas: But in the interest of patient safety, this principle shall always be followed.
The focus here should not be on stubbornly following rules that have already been laid down, but it should be replaced by the concept of regulatory thinking in design, development and production.
„Regulatory Thinking describes a strategic regulatory approach developed by the author together with the 4C accelerator operated by the Medical Innovation Incubator and the Foundation for Medical Innovation Tübingen and << ETHzürich >> as a regulatory strategy that knows existing rules, regulations, standards and norms, adapts them to new challenges and applies them in the spirit of the <<original idea>>”.
Dietmar Schaffarczyk, ETH Zurich